 -
Volume 149,
Issue 5,
2003
-
Volume 149,
Issue 5,
2003
Volume 149, Issue 5, 2003
- Pathogens And Pathogenicity
-
-
-
SseA is a chaperone for the SseB and SseD translocon components of the Salmonella pathogenicity-island-2-encoded type III secretion system
More LessThe type III secretion system (TTSS) encoded by the Salmonella pathogenicity island 2 (SPI-2) is required for bacterial replication inside macrophages and for systemic infection in mice. Many TTSS secreted proteins, including effectors and components of the translocon, require chaperones which promote their stability, prevent their premature interactions or facilitate their secretion. In this study, the function of the first gene (sseA) of one of the SPI-2 operons (sseA–G) was investigated. This operon includes genes that encode translocon components (SseB, SseC and SseD), translocated proteins (SseF and SseG) and putative chaperones (SscA and SscB). sseA encodes a 12·5 kDa protein with a C-terminal region with the potential to form a coiled-coil structure, but no sequence similarity to other proteins. Mutation of sseA results in severe virulence attenuation and an intracellular replication defect. It is shown here that SseA is not a secreted protein, but is required for SPI-2-dependent translocation of two effector proteins (SifA and PipB). Furthermore, the translocon components SseB and SseD were not detected in an sseA mutant strain. By using a yeast two-hybrid assay and column binding experiments, it is demonstrated that SseA interacts directly with SseB and SseD. These results indicate that SseA is a chaperone for SseB and SseD. The inability of an sseA mutant to assemble the SPI-2 TTSS translocon accounts for its high level of virulence attenuation in vivo. To the authors' knowledge, this is the first chaperone described for the SPI-2 TTSS.
-
-
-
-
Immunological and genetic characterization of Borrelia burgdorferi BapA and EppA proteins
More LessA large majority of examined Lyme disease spirochaete isolates were demonstrated to contain one or both of the paralogous genes bapA and eppA. Immunological analyses of serum samples collected from infected patients coupled with comparative sequence analyses indicated that bapA gene sequences are quite stable but the encoded proteins do not provoke a strong immune response in most individuals. Conversely, EppA proteins are much more antigenic but vary widely in sequence between different bacteria. Considerable evidence of insertion, deletion and other mutations within eppA genes was observed. A number of significant recombination events were also found to have occurred in regions flanking bapA genes, while the genes themselves rarely exhibited evidence of mutation, suggesting strong selective pressure to maintain BapA sequences within narrow limits. Data from these and other studies suggest important roles for BapA and EppA during the Borrelia burgdorferi infectious cycle.
-
-
-
Modification of the signal sequence cleavage site of listeriolysin O does not affect protein secretion but impairs the virulence of Listeria monocytogenes
More LessListeriolysin O (LLO, hly-encoded), a major virulence factor secreted by the bacterial pathogen Listeria monocytogenes, is synthesized as a precursor of 529 residues. To impair LLO secretion, the four residues of the predicted signal sequence cleavage site (EA-KD) were deleted and the mutant LLO protein was expressed in a hly-negative derivative of L. monocytogenes. Unexpectedly, the mutant protein was secreted in normal amounts in the culture supernatant and was fully haemolytic. N-terminal sequencing of the secreted LLO molecule revealed that N-terminal processing of the preprotein occurred three residues downstream of the natural cleavage site. L. monocytogenes expressing this truncated LLO showed a reduced capacity to disrupt the phagosomal membranes of bone marrow macrophages and of hepatocytes; and the mutant strain showed a 100-fold decrease in virulence in the mouse model. These results suggest that the first N-terminal residues of mature LLO participate directly in phagosomal escape and bacterial infection.
-
- Physiology
-
-
-
Only one catalase, katG, is detectable in Rhizobium etli, and is encoded along with the regulator OxyR on a plasmid replicon
More LessThe plasmid-borne Rhizobium etli katG gene encodes a dual-function catalase-peroxidase (KatG) (EC 1.11.1.7) that is inducible and heat-labile. In contrast to other rhizobia, katG was shown to be solely responsible for catalase and peroxidase activity in R. etli. An R. etli mutant that did not express catalase activity exhibited increased sensitivity to hydrogen peroxide (H2O2). Pre-exposure to a sublethal concentration of H2O2 allowed R. etli to adapt and survive subsequent exposure to higher concentrations of H2O2. Based on a multiple sequence alignment with other catalase-peroxidases, it was found that the catalytic domains of the R. etli KatG protein had three large insertions, two of which were typical of KatG proteins. Like the katG gene of Escherichia coli, the R. etli katG gene was induced by H2O2 and was important in sustaining the exponential growth rate. In R. etli, KatG catalase-peroxidase activity is induced eightfold in minimal medium during stationary phase. It was shown that KatG catalase-peroxidase is not essential for nodulation and nitrogen fixation in symbiosis with Phaseolus vulgaris, although bacteroid proteome analysis indicated an alternative compensatory mechanism for the oxidative protection of R. etli in symbiosis. Next to, and divergently transcribed from the catalase promoter, an ORF encoding the regulator OxyR was found; this is the first plasmid-encoded oxyR gene described so far. Additionally, the katG promoter region contained sequence motifs characteristic of OxyR binding sites, suggesting a possible regulatory mechanism for katG expression.
-
-
-
-
Regulation of expression of the cyanide-insensitive terminal oxidase in Pseudomonas aeruginosa
More LessThe regulation of the cyanide-insensitive oxidase (CIO) in Pseudomonas aeruginosa, a bacterium that can synthesize HCN, is reported. The expression of a cioA–lacZ transcriptional fusion, CioA protein levels and CIO activity were low in exponential phase but induced about fivefold upon entry into stationary phase. Varying the O2 transfer coefficient from 11·5 h−1 to 87·4 h−1 had no effect on CIO expression and no correlation was observed between CIO induction and the dissolved O2 levels in the growth medium. However, a mutant deleted for the O2-sensitive transcriptional regulator ANR derepressed CIO expression in an O2-sensitive manner, with the highest induction occurring under low-O2 conditions. Therefore, CIO expression can respond to a signal generated by low O2 levels, but this response is normally kept in check by ANR repression. ANR may play an important role in preventing overexpression of the CIO in relation to other terminal oxidases. A component present in spent culture medium was able to induce CIO expression. However, experiments with purified N-butanoyl-l-homoserine lactone or N-(3-oxododecanoyl)homoserine lactone ruled out a role for these quorum-sensing molecules in the control of CIO expression. Cyanide was a potent inducer of the CIO at physiologically relevant concentrations and experiments using spent culture medium from a ΔhcnB mutant, which is unable to synthesize cyanide, showed that cyanide was the inducing factor present in P. aeruginosa spent culture medium. However, the finding that in a ΔhcnB mutant cioA–lacZ expression was induced normally upon entry into stationary phase indicated that cyanide was not the endogenous inducer of the terminal oxidase. The authors suggest that the failure of O2 to have an effect on CIO expression in the wild-type can be explained either by the requirement for an additional, stationary-phase-specific inducing signal or by the loss of an exponential-phase-specific repressing signal.
-
-
-
An inducible tellurite-resistance operon in Proteus mirabilis
More LessTellurite resistance (Ter) is widespread in nature and it is shown here that the natural resistance of Proteus mirabilis to tellurite is due to a chromosomally located orthologue of plasmid-borne ter genes found in enteric bacteria. The P. mirabilis ter locus (terZABCDE) was identified in a screen of Tn5lacZ-generated mutants of which one contained an insertion in terC. The P. mirabilis terC mutant displayed increased susceptibility to tellurite (Tes) and complementation with terC carried on a multicopy plasmid restored high-level Ter. Primer extension analysis revealed a single transcriptional start site upstream of terZ, but only with RNA harvested from bacteria grown in the presence of tellurite. Northern blotting and reverse transcriptase-PCR (RT-PCR) analyses confirmed that the ter operon was inducible by tellurite and to a lesser extent by oxidative stress inducers such as hydrogen peroxide and methyl viologen (paraquat). Direct and inverted repeat sequences were identified in the ter promoter region as well as motifs upstream of the −35 hexamer that resembled OxyR-binding sequences. Finally, the 390 bp intergenic promoter region located between orf3 and terZ showed no DNA sequence identity with any other published ter sequences, whereas terZABCDE genes exhibited 73–85 % DNA sequence identity. The ter operon was present in all clinical isolates of P. mirabilis and Proteus vulgaris tested and is inferred for Morganella and Providencia spp. based on screening for high level Ter and preliminary PCR analysis. Thus, a chromosomally located inducible tellurite resistance operon appears to be a common feature of the genus Proteus.
-
- Plant-Microbe Interactions
-
-
-
Alginate gene expression by Pseudomonas syringae pv. tomato DC3000 in host and non-host plants
More LessPseudomonas syringae produces the exopolysaccharide alginate, a copolymer of mannuronic and guluronic acid. Although alginate has been isolated from plants infected by P. syringae, the signals and timing of alginate gene expression in planta have not been described. In this study, an algD : : uidA transcriptional fusion, designated pDCalgDP, was constructed and used to monitor alginate gene expression in host and non-host plants inoculated with P. syringae pv. tomato DC3000. When leaves of susceptible collard plants were spray-inoculated with DC3000(pDCalgDP), algD was activated within 72 h post-inoculation (p.i.) and was associated with the development of water-soaked lesions. In leaves of the susceptible tomato cv. Rio Grande-PtoS, algD activity was lower than in collard and was not associated with water-soaking. The expression of algD was also monitored in leaves of tomato cv. Rio Grande-PtoR, which is resistant to P. syringae pv. tomato DC3000. Within 12 h p.i., a microscopic hypersensitive response (micro-HR) was observed in Rio Grande-PtoR leaves spray-inoculated with P. syringae pv. tomato DC3000(pDCalgDP). As the HR progressed, histochemical staining indicated that individual bacterial cells on the surface of resistant tomato leaves were expressing algD. These results indicate that algD is expressed in both susceptible (e.g. collard, tomato) and resistant (Rio Grande-PtoR) host plants. The expression of algD in an incompatible host–pathogen interaction was further explored by monitoring transcriptional activity in leaves of tobacco, which is not a host for P. syringae pv. tomato. In tobacco inoculated with DC3000(pDCalgDP), an HR was evident within 12 h p.i., and algD expression was evident within 8-12 h p.i. However, when tobacco was inoculated with an hrcC mutant of DC3000, the HR did not occur and algD expression was substantially lower. These results suggest that signals that precede the HR may stimulate alginate gene expression in P. syringae. Histochemical staining with nitro blue tetrazolium indicated that the superoxide anion (
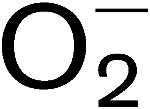 ) is a signal for algD activation in planta. This study indicates that algD is expressed when P. syringae attempts to colonize both susceptible and resistant plant hosts.
) is a signal for algD activation in planta. This study indicates that algD is expressed when P. syringae attempts to colonize both susceptible and resistant plant hosts.
-
-
- Theoretical Microbiology
-
-
-
A computer investigation of chemically mediated detachment in bacterial biofilms
More LessA three-dimensional computer model was used to evaluate the effect of chemically mediated detachment on biofilm development in a negligible-shear environment. The model, BacLAB, combines conventional diffusion-reaction equations for chemicals with a cellular automata algorithm to simulate bacterial growth, movement and detachment. BacLAB simulates the life cycle of a bacterial biofilm from its initial colonization of a surface to the development of a mature biofilm with cell areal densities comparable to those in the laboratory. A base model founded on well established transport equations that are easily adaptable to investigate conjectures at the biological level has been created. In this study, the conjecture of a detachment mechanism involving a bacterially produced chemical detachment factor in which high local concentrations of this detachment factor cause the bacteria to detach from the biofilm was examined. The results show that the often observed ‘mushroom’-shaped structure can occur if detachment events create voids so that the remaining attached cells look like mushrooms.
-
-
Volumes and issues
-
Volume 170 (2024)
-
Volume 169 (2023)
-
Volume 168 (2022)
-
Volume 167 (2021)
-
Volume 166 (2020)
-
Volume 165 (2019)
-
Volume 164 (2018)
-
Volume 163 (2017)
-
Volume 162 (2016)
-
Volume 161 (2015)
-
Volume 160 (2014)
-
Volume 159 (2013)
-
Volume 158 (2012)
-
Volume 157 (2011)
-
Volume 156 (2010)
-
Volume 155 (2009)
-
Volume 154 (2008)
-
Volume 153 (2007)
-
Volume 152 (2006)
-
Volume 151 (2005)
-
Volume 150 (2004)
-
Volume 149 (2003)
-
Volume 148 (2002)
-
Volume 147 (2001)
-
Volume 146 (2000)
-
Volume 145 (1999)
-
Volume 144 (1998)
-
Volume 143 (1997)
-
Volume 142 (1996)
-
Volume 141 (1995)
-
Volume 140 (1994)
-
Volume 139 (1993)
-
Volume 138 (1992)
-
Volume 137 (1991)
-
Volume 136 (1990)
-
Volume 135 (1989)
-
Volume 134 (1988)
-
Volume 133 (1987)
-
Volume 132 (1986)
-
Volume 131 (1985)
-
Volume 130 (1984)
-
Volume 129 (1983)
-
Volume 128 (1982)
-
Volume 127 (1981)
-
Volume 126 (1981)
-
Volume 125 (1981)
-
Volume 124 (1981)
-
Volume 123 (1981)
-
Volume 122 (1981)
-
Volume 121 (1980)
-
Volume 120 (1980)
-
Volume 119 (1980)
-
Volume 118 (1980)
-
Volume 117 (1980)
-
Volume 116 (1980)
-
Volume 115 (1979)
-
Volume 114 (1979)
-
Volume 113 (1979)
-
Volume 112 (1979)
-
Volume 111 (1979)
-
Volume 110 (1979)
-
Volume 109 (1978)
-
Volume 108 (1978)
-
Volume 107 (1978)
-
Volume 106 (1978)
-
Volume 105 (1978)
-
Volume 104 (1978)
-
Volume 103 (1977)
-
Volume 102 (1977)
-
Volume 101 (1977)
-
Volume 100 (1977)
-
Volume 99 (1977)
-
Volume 98 (1977)
-
Volume 97 (1976)
-
Volume 96 (1976)
-
Volume 95 (1976)
-
Volume 94 (1976)
-
Volume 93 (1976)
-
Volume 92 (1976)
-
Volume 91 (1975)
-
Volume 90 (1975)
-
Volume 89 (1975)
-
Volume 88 (1975)
-
Volume 87 (1975)
-
Volume 86 (1975)
-
Volume 85 (1974)
-
Volume 84 (1974)
-
Volume 83 (1974)
-
Volume 82 (1974)
-
Volume 81 (1974)
-
Volume 80 (1974)
-
Volume 79 (1973)
-
Volume 78 (1973)
-
Volume 77 (1973)
-
Volume 76 (1973)
-
Volume 75 (1973)
-
Volume 74 (1973)
-
Volume 73 (1972)
-
Volume 72 (1972)
-
Volume 71 (1972)
-
Volume 70 (1972)
-
Volume 69 (1971)
-
Volume 68 (1971)
-
Volume 67 (1971)
-
Volume 66 (1971)
-
Volume 65 (1971)
-
Volume 64 (1970)
-
Volume 63 (1970)
-
Volume 62 (1970)
-
Volume 61 (1970)
-
Volume 60 (1970)
-
Volume 59 (1969)
-
Volume 58 (1969)
-
Volume 57 (1969)
-
Volume 56 (1969)
-
Volume 55 (1969)
-
Volume 54 (1968)
-
Volume 53 (1968)
-
Volume 52 (1968)
-
Volume 51 (1968)
-
Volume 50 (1968)
-
Volume 49 (1967)
-
Volume 48 (1967)
-
Volume 47 (1967)
-
Volume 46 (1967)
-
Volume 45 (1966)
-
Volume 44 (1966)
-
Volume 43 (1966)
-
Volume 42 (1966)
-
Volume 41 (1965)
-
Volume 40 (1965)
-
Volume 39 (1965)
-
Volume 38 (1965)
-
Volume 37 (1964)
-
Volume 36 (1964)
-
Volume 35 (1964)
-
Volume 34 (1964)
-
Volume 33 (1963)
-
Volume 32 (1963)
-
Volume 31 (1963)
-
Volume 30 (1963)
-
Volume 29 (1962)
-
Volume 28 (1962)
-
Volume 27 (1962)
-
Volume 26 (1961)
-
Volume 25 (1961)
-
Volume 24 (1961)
-
Volume 23 (1960)
-
Volume 22 (1960)
-
Volume 21 (1959)
-
Volume 20 (1959)
-
Volume 19 (1958)
-
Volume 18 (1958)
-
Volume 17 (1957)
-
Volume 16 (1957)
-
Volume 15 (1956)
-
Volume 14 (1956)
-
Volume 13 (1955)
-
Volume 12 (1955)
-
Volume 11 (1954)
-
Volume 10 (1954)
-
Volume 9 (1953)
-
Volume 8 (1953)
-
Volume 7 (1952)
-
Volume 6 (1952)
-
Volume 5 (1951)
-
Volume 4 (1950)
-
Volume 3 (1949)
-
Volume 2 (1948)
-
Volume 1 (1947)
Most Read This Month


