
Full text loading...
Mycobacterium bovis is a slow-growing (16–20 h generation time), Gram-positive and acid-fast bacterium member of the Mycobacterium tuberculosis complex pathogen group (MTBC). They are characterized by a complex, protective cell wall containing mycolic acids. The MTBCs are the causative agents of tuberculosis (TB). Following initial infection, subsequent pathological changes, and the progress of infection depend on the interplay between host defence mechanisms and mycobacterial virulence factors and the balance between the immunologic protective responses and the damaging inflammatory processes. Progression of the disease is characterized by the formation of typical caseous tuberculous granuloma (inflammatory mononuclear cell aggregates) because of the host's immune response to infection. The transmission and epidemiology of Mycobacterium bovis are complex and vary depending on the situation and ecosystem. In the UK, the spread of BTB in the UK cattle herd can occur by transmitting the disease from cattle to cattle and between badgers but also between badgers and cattle. The disease is thought to be primarily a respiratory disease with spread between individuals through mechanisms such as coughing or transfer of bacteria in respiratory secretions. It is also thought that environmental contamination may also lead to some transmission. The protective cell wall of the organism is believed to allow the organism to survive outside an animal host, which can then transfer to new hosts following subsequent environmental exposure. In some situations, ingestion of pathogens in food can lead to infection. The relative contribution of these routes and precise transmission mechanisms needs to be better understood.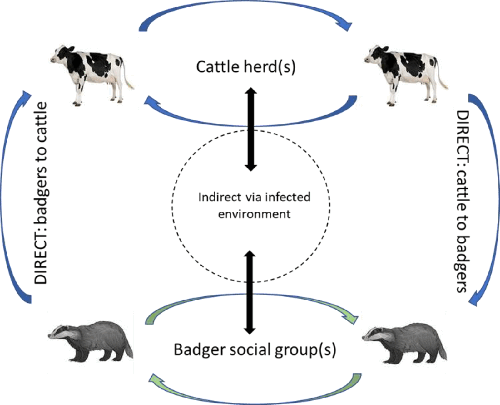
Mycobacterium bovis belongs to the Mycobacterium tuberculosis complex pathogen group (MTBC), which includes M. tuberculosis . These organisms cause tuberculosis (TB), a disease characterized by the formation of tubercles and caseous necrosis in the lungs. M. bovis is the leading cause of bovine tuberculosis (bTB) in cattle and other domestic and wild animals. It also causes disease in humans (zoonotic TB). Other MTBC pathogens like M. caprae or M. orygis can also cause bTB. However, M. bovis is the focus of this review. In addition to direct health consequences, bTB has a significant global economic impact in developed and developing countries.