
Full text loading...
Sub-MICs of ciprofloxacin result in DNA breakage, triggering DNA damage stress and the SOS stress response. This, in turn, induces changes in regulatory factors and the translation machinery. Modifications to regulatory components, including MvaT, RpoS and RpoB, lead to the upregulation (depicted in green) of efflux pumps such as MexEF-OprN and the downregulation (depicted in red) of porins. Additionally, there are alterations in the expression of virulence factors. Concurrently, ciprofloxacin affects metabolic processes, shortens cell lengths and induces the formation of multinucleated filaments due to disruptions in cell replication.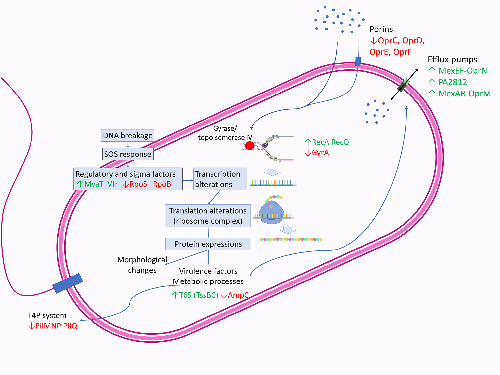
Understanding the evolution of antibiotic resistance is important for combating drug-resistant bacteria. In this work, we investigated the adaptive response of Pseudomonas aeruginosa to ciprofloxacin. Ciprofloxacin-susceptible P. aeruginosa ATCC 9027, CIP-E1 (P. aeruginosa ATCC 9027 exposed to ciprofloxacin for 14 days) and CIP-E2 (CIP-E1 cultured in antibiotic-free broth for 10 days) were compared. Phenotypic responses including cell morphology, antibiotic susceptibility, and production of pyoverdine, pyocyanin and rhamnolipid were assessed. Proteomic responses were evaluated using comparative iTRAQ labelling LC-MS/MS to identify differentially expressed proteins (DEPs). Expression of associated genes coding for notable DEPs and their related regulatory genes were checked using quantitative reverse transcriptase PCR. CIP-E1 displayed a heterogeneous morphology, featuring both filamentous cells and cells with reduced length and width. By contrast, although filaments were not present, CIP-E2 still exhibited size reduction. Considering the MIC values, ciprofloxacin-exposed strains developed resistance to fluoroquinolone antibiotics but maintained susceptibility to other antibiotic classes, except for carbapenems. Pyoverdine and pyocyanin production showed insignificant decreases, whereas there was a significant decrease in rhamnolipid production. A total of 1039 proteins were identified, of which approximately 25 % were DEPs. In general, there were more downregulated proteins than upregulated proteins. Noted changes included decreased OprD and PilP, and increased MexEF-OprN, MvaT and Vfr, as well as proteins of ribosome machinery and metabolism clusters. Gene expression analysis confirmed the proteomic data and indicated the downregulation of rpoB and rpoS. In summary, the response to CIP involved approximately a quarter of the proteome, primarily associated with ribosome machinery and metabolic processes. Potential targets for bacterial interference encompassed outer membrane proteins and global regulators, such as MvaT.