
Full text loading...
The amoeba Acanthamoeba castellanii generated both luminol- and lucigenin-enhanced chemiluminescence upon addition of the respiratory inhibitor sodium cyanide, but not upon the addition of sodium azide. Photon emission in the presence of lucigenin was three- to fourfold greater than that measured in the presence of luminol, but both forms of chemiluminescence were strictly dependent upon the presence of O2, indicating the requirement for oxidative reactions in these processes. Lucigenin-chemilununescence measured during the phagocytosis of latex beads under identical conditions was, however, barely detectable above background levels. Cyanide similarly induced the formation of 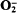 , as indicated by the stimulation of superoxide-dismutase-inhibitable cytochrome c reduction, and the rate of production was similar to that previously observed during phagocytosis of latex particles or yeasts by these cells. Thus, in view of the similar rates of
, as indicated by the stimulation of superoxide-dismutase-inhibitable cytochrome c reduction, and the rate of production was similar to that previously observed during phagocytosis of latex particles or yeasts by these cells. Thus, in view of the similar rates of 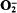 production during cyanide exposure or phagocytosis, but disparate rates of lucigenin-chemiluminescence, these two treatments must activate different molecular processes leading to reactive oxidant production. The rates of cyanide-stimulated lucigenin-chemiluminescence were directly proportional to the O2 tensions in the medium from 0 to 300 μM, indicating that the rates of free-radical-generating reactions were directly related to the oxygen tensions in the environment. The superoxide dismutase inhibitor diethyldithiocarbamate similarly stimulated lucigenin-chemiluminescence, with photon emission again being dependent upon O2 tensions in the range 0–320 μM. A mechanism by which cells may limit
production during cyanide exposure or phagocytosis, but disparate rates of lucigenin-chemiluminescence, these two treatments must activate different molecular processes leading to reactive oxidant production. The rates of cyanide-stimulated lucigenin-chemiluminescence were directly proportional to the O2 tensions in the medium from 0 to 300 μM, indicating that the rates of free-radical-generating reactions were directly related to the oxygen tensions in the environment. The superoxide dismutase inhibitor diethyldithiocarbamate similarly stimulated lucigenin-chemiluminescence, with photon emission again being dependent upon O2 tensions in the range 0–320 μM. A mechanism by which cells may limit 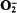 generating reactions, and so reduce damaging free radical reactions, was observed when anaerobic suspensions were reaerated. These data indicate that oxidative stress and phagocytosis provide two intracellular sources of free-radical generating reactions in these cells.
generating reactions, and so reduce damaging free radical reactions, was observed when anaerobic suspensions were reaerated. These data indicate that oxidative stress and phagocytosis provide two intracellular sources of free-radical generating reactions in these cells.